For your (pre)launch and full commercialization of specialty medicines in the Netherlands, Belgium and Luxembourg.
Bio

Marco Fossatelli is an experienced leader in the pharmaceutical and biotechnology industry with a strong scientific and commercial background. He holds a PhD in organic chemistry from Utrecht University and began his career as a researcher in Italy. He soon transitioned to the commercial side of healthcare, where he combined his scientific expertise with entrepreneurship and leadership.
Throughout his career, Marco has held various senior positions at international pharmaceutical companies, including Altana Pharma, Shire, Raptor Pharmaceuticals and Alnylam Pharmaceuticals. He has extensive experience in successfully launching innovative medicines, particularly in the field of rare diseases. He played a key role in multiple product launches in the Netherlands and the broader Benelux region. His focus extends beyond market access and medical education to close collaboration with physicians, patient organizations, and healthcare authorities.
Marco has now started his own company, where he aims to use his experience and passion for biotech to support companies in bringing their innovative therapies to patients in the Netherlands, Belgium and Luxembourg.
For your (pre)launch and commercialization of specialty medicines in the Netherlands, Belgium and Luxembourg.
What: My activities
5 steps towards successful commercialization
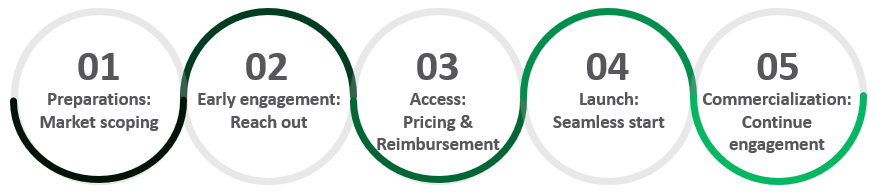
Engage with me for one, some or all steps!
How: The way I work
- Very dedicated to the community and company I work for
- Strong scientific focus
- Strong commercial focus
- Good communicator & storyteller
- Autonomous, collaborative, transparent
- Extensive knowledge of compliance and legal aspects
- Committed to creating a positive societal impact
Some recent activities
– Successfully launched 4 products in a new class in the Benelux at a growing biotech, the first as a ‘lone wolf’ and the last two as General Manager of a large site
– Support for global launch preparations of a soon to be approved innovative treatment
Support for P&R negotiations for revolutionary gene therapy
– Help a biotech start-up with their product positioning and commercial thinking
Passionate about bringing science to patients
I started my career in organic chemistry, where I synthesized many molecules that were tested as a new treatment against serious diseases. Later I switched to commercial roles in the pharmaceutical industry, helping to bring these innovations to patients. My focus here has been specialty medicines, often with an interesting scientific foundation, complex pricing and reimbursement procedures and a high need both from physicians and patients. I still thoroughly enjoy this work.
.




